Table of Contents
Lawrence Livermore National Laboratory (LLNL) researchers identified a first-of-its-kind carbon dioxide-equivalent polymer recoverable from high-pressure conditions on January 27, 2026. Led by scientist Stanimir Bonev, the team published their findings in Communications Chemistry, revealing a recipe for new energetic materials useful in propellants and explosives.
Breakthrough in High-Pressure Material Recovery
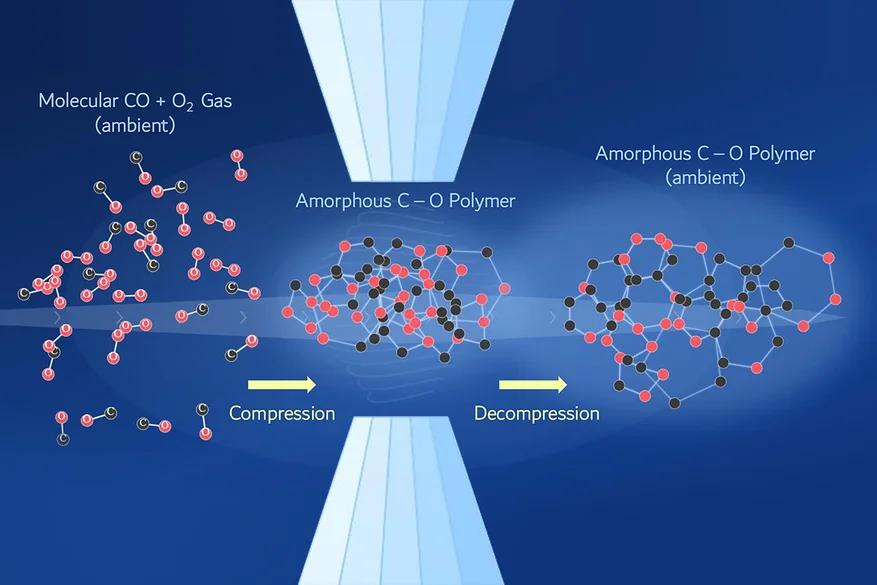
The innovation centers on transforming fleeting high-pressure atomic arrangements into stable materials under ambient conditions. Traditional carbon dioxide under compression forms dense structures, but they revert upon pressure release. LLNL's approach locks atoms into a covalently bonded network, creating a polymeric form that stores significantly more energy per unit mass or volume than ordinary CO2.
Key Methodology: Simulations and Mixture Compression
Researchers combined quantum molecular dynamics simulations with large-scale machine-learning models to predict polymer formation pathways. They explored broad pressure and temperature ranges, identifying optimal conditions. The pivotal strategy: compressing a mixture of carbon monoxide (CO) and oxygen (O2) instead of pure CO2. This lowers required pressures, enables flexible reactions, and favors amorphous solids over crystals for better stability post-decompression.
- Quantum simulations modeled high-pressure behavior and release dynamics.
- Machine learning accelerated exploration of conditions.
- CO + O2 mixture forms carbon-carbon bonds, stabilizing the structure.
Physical Explanation of Stability
Carbon-carbon bonds emerge readily in the mixture, forming a distinct structure that persists after pressure release. Amorphous nature avoids crystal defects that destabilize upon decompression. Bonev explained: "A polymeric form of carbon dioxide stores far more energy... representing a high-energy-density material." This distinguishes it from transient high-pressure phases.
Potential Applications and Broader Impact
Energetic materials from this polymer could advance propellants and explosives with superior energy density. The method extends to light-element systems like carbon, oxygen, nitrogen, hydrogen, potentially yielding new functional materials. LLNL provides a concrete experimental target: compress CO-O2 mixtures under specified conditions to synthesize and recover the polymer.
Comparison to Prior High-Pressure Research
Unlike pure CO2 studies, which fail at ambient recovery, the mixture approach succeeds at lower pressures. Previous efforts yielded only transient phases; this identifies a stable, amorphous polymer. No direct competitors match this CO2-equivalent recoverability, positioning LLNL's work as pioneering in energetic material design.
Experimental validation could follow simulations, with machine-learning guidance optimizing synthesis. Related LLNL code developments for non-equilibrium simulations, like foam targets at National Ignition Facility, suggest synergies for atomistic-hydrodynamic modeling. Success here may enable materials releasing energy controllably, advancing defense and aerospace applications backed by the polymer's predicted high density.